Process for continuous production of controlled-release microspheres of fairly even size
This process is consist of four inline unit operations as size-regulated microsphere formulation; sedimentation aided microspheres solidification; rinsing for removing surfactants and salts; and formulation prior to lyophilization. Our home-made sterilized pilot scale production system is able to produce 25 g to 100 g microspheres (equivalent to 500 to 2000 shots of injections if one dose contains 50 mg microspheres). This 25 g to 100 g scale-up production requires no charge of apparatus and operation parameters except extending the operation time.
Our pilot scale continuous production system mounted in a sterile carbin

Aqueous-aqueous "emulsion"-aided pre-formulation for preserving protein conformation
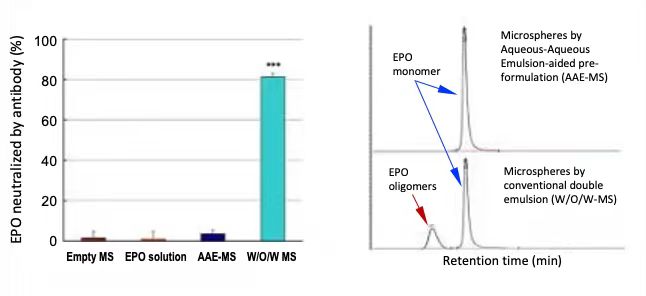
Loading proteins into controlled-release microspheres for prolonged efficacy per injection encounters immunogenicity issue caused by protein denaturation during the formulation process involving organic solvent. To avoid protein denaturing, we developed a pre-formulation method by which delicate proteins can be immobilized in the matrix of polysaccharide fine particles prior to micro encapsulation. The protein to be formulated is added in an aqueous solution of a polysaccharide and dispersed in an aqueous continuous phase containing polyethylene glycol (PEG). The polysaccharide dispersed phases are stabilized by zeta potential and the protein is partitioned inside the polysaccharide phase preferentially. This "emulsion" is then lyophilized, followed by washing off the PEG continuous phase using organic solvents. Finally, the protein-loaded polysaccharide particles are suspended in a biodegradable polymer solution and loaded in the machine as above to produce microspheres. This pre-formulation method was confirmed by formulating erythropoietin (EPO) into biweekly injecting microspheres through our aqueous-aqueous emulsion (AAE) aided microencapsulation and conventional double emulsion (W/O/W) microencapsulation and injecting to monkeys for 2 months period, followed by titrating anti-EPO antibody neutralized EPO and SEC-HPLC measurement of aggregated EPO which were recovered from the two microsphere formulations.
Anti-EPO antibody titer and SEC-HPLC chat of EPO in serum of monkeys received microsphere injections for 2 months
|

