Extrusion-freezing 3D printing for highly efficient production of pharmaceutical microneedle patches
Unlike cosmetic microneedle patches (with needle tips below 0.3 mm in length), micrnneedle patches for transdermal delivery of lipophobic medicines have to ensure sufficient bioavailability and defined absorption rate, for which the length of needle tips should best be over 0.8 mm. This length will cause some difficulties for demolding of casted microneedle patches without needle tip breakage if conventional molding methods are used to fabricate microneedle patches. Moreover, molding methods consist of multiple steps when applied to produce pharmaceutical microneedle patches for which sophisticated Robot systems are required for automatic manufacture.
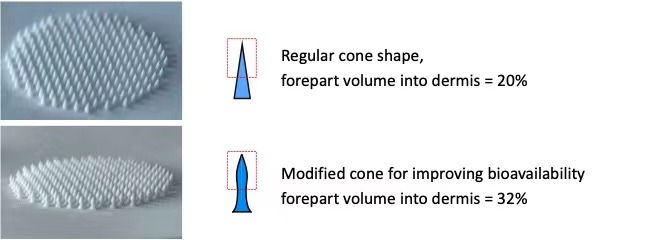
To address this issues, our scientists invented a unique in which the microneedle-forming aqueous solution of polymers are extruded through a nozzle with an array of micro holes onto a pre-frozen back sheet to form a microneedle array of hundreds of needle tips in seconds. This production engineering needs no sophisticated robot system beyond moving injecting pumps and is easily scaled up for massive manufacture by simply increasing the numbers of the injection pumps. Furthermore, our 3D printing method enables microneedles of enlarged front arrow and shrunk waist be fabricated by which transdermal bioavailability of protein and peptide medicines can be substantially improved.
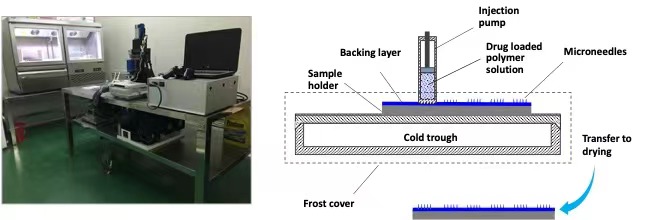
The images of a home-made 3D printing system, its working mechanism, as well as examples printed micro needle patches are shown below. A pilot scale 3D printing system for producing microneedle patches suitable for clinical trials I and II is under designing and fabricating.
Home-made extrusion-freezing 3D printing system Working mechanism of extrusion-freezing 3D printing
Microneedles of regular and modified cone shape produced by extrusion-freezing 3D printing
Hydrogel-forming microneedle patches by freeze-thaw cross-linking for efficient and safe transdermal drug delivery
Compared with dissolvable microneedles frequently reported in the literature, hydrogel-forming microneedles offer two meaningful advantages: the issue of skin deposition of needle tip materials which prohibit frequent application no longer exist; medication can be terminated by pulling out the entire drug-loaded microneedle patch whenever overdose (such as insulin) is in concern. To avoid dissolution by the interstitial fluid of the dermis tissue and achieve swelling-driven release, however, the hydrophilic polymer chains of microneedle matrix have to be cross-linked through chemical reactions, which results in difficulties to load insulin in the microneedle tips. Our scientists replaced the chemical cross-linking with a convenient and protein-friendly freeze-thaw treatment during which the polymeric chains form sub-micron-sized crystalline domains as the cross-linking junctions.
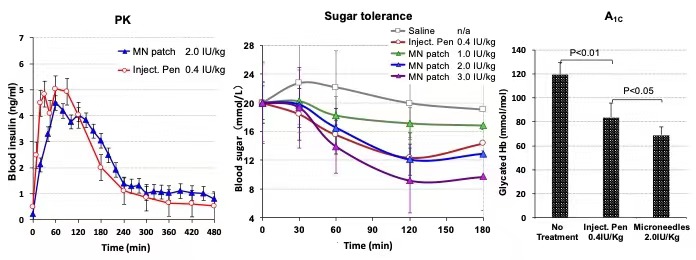
We loaded insulin lispro in the above hydrogel-forming microneedle patches (made by molding method) and found satisfied results in pig models. The TMAX after applying the patches to diabetic pigs was only 18 min behind and the overall AUC was 20% (twice as that of Pfizer's insulin inhale) as compared with of injection pens of the same insulin.
PK/PD of insulin loaded in pig model
|

